Difference between revisions of "Mitocondrial pyruvate metabolism"
| Line 13: | Line 13: | ||
<center><math> v=K_1A-K_2B</math></center><br> | <center><math> v=K_1A-K_2B</math></center><br> | ||
<center><math> v=k_1A \left(1-\frac{K_2B}{K_1A} \right)</math></center><br> | <center><math> v=k_1A \left(1-\frac{K_2B}{K_1A} \right)</math></center><br> | ||
| + | Considering <math>K_{eq} = \frac{K_1}{K_2}</math> and <math>\tau = \frac{P_1P_2 \ldots}{S_1S_2 \ldots}</math> we have, | ||
<center><math> v=k_1A \left(1-\frac{\tau}{K_{eq}} \right)</math></center><br> | <center><math> v=k_1A \left(1-\frac{\tau}{K_{eq}} \right)</math></center><br> | ||
Revision as of 10:32, 12 May 2014
Mitocondrial pyruvate metabolism(MPM) is an enzyme that generates ATP form pyruvate.
Chemical reaction
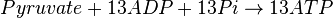
Rate equation
- Chemical reactions proceed to equilibrium within closed systems. For a simple reaction
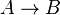 it is defined as
it is defined as ![K_{eq} = \frac{[B]_{eq}}{[A]_{eq}}](/wiki/images/math/a/c/8/ac844b70f8f7c5f1d91accb00061a1ea.png) where forward and reverse rates are equal.
where forward and reverse rates are equal. - Equilibrium is not reached in open system due to influx and outflux. Mass action ratio[1]
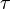 for
for 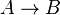 reaction is defined as
reaction is defined as ![\tau = \frac{[B]_{ob}}{[A]_{ob}}](/wiki/images/math/8/0/5/8050a810298db95df036d87c2e79f1ea.png) where subscript ob represents observable at a given point.
where subscript ob represents observable at a given point. - Deviation from equilibrium is measured with Disequilibrium constant
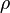 as
as 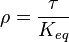
- Given the simple uni molecular reaction
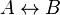 the mass action equation can be modified as
the mass action equation can be modified as
Considering 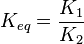 and
and 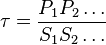 we have,
we have,
Constant flux is used where 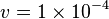 is considered.
is considered.
Parameter values
| Parameter | Value | Organism | Remarks |
|---|---|---|---|
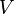
|
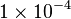 [2] [2]
|
HeLa cell line | Constant flux |
References
- ↑ Hess B. and Brand K. (1965), Enzymes and metabolite profiles. In Control of energy metabolism. III. Ed. B. Chance, R. K. Estabrook and J. R. Williamson. New York: Academic Press
- ↑ Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S et al (2011) Modeling cancer glycolysis. Biochim Biophys Acta 1807:755–767 (doi)