Difference between revisions of "Mitocondrial pyruvate metabolism"
(→Rate equation) |
|||
| Line 7: | Line 7: | ||
==Rate equation== | ==Rate equation== | ||
| + | *Chemical reactions proceed to equilibrium within closed systems. For a simple reaction <math>A \rightarrow B</math> it is defined as <math>K_{eq} = \frac{[B]_{eq}}{[A]_{eq}}</math> where forward and reverse rates are equal. | ||
Constant flux is used where <math> v = 1 \times 10^{-4}</math> is considered. | Constant flux is used where <math> v = 1 \times 10^{-4}</math> is considered. | ||
| − | |||
==Parameter values== | ==Parameter values== | ||
Revision as of 10:13, 12 May 2014
Mitocondrial pyruvate metabolism(MPM) is an enzyme that generates ATP form pyruvate.
Chemical reaction
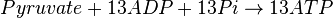
Rate equation
- Chemical reactions proceed to equilibrium within closed systems. For a simple reaction
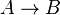 it is defined as
it is defined as ![K_{eq} = \frac{[B]_{eq}}{[A]_{eq}}](/wiki/images/math/a/c/8/ac844b70f8f7c5f1d91accb00061a1ea.png) where forward and reverse rates are equal.
where forward and reverse rates are equal.
Constant flux is used where 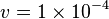 is considered.
is considered.
Parameter values
| Parameter | Value | Organism | Remarks |
|---|---|---|---|
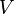
|
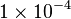 [1] [1]
|
HeLa cell line | Constant flux |