Difference between revisions of "Phosphoglucomutase"
| Line 8: | Line 8: | ||
==Rate equation== | ==Rate equation== | ||
Mono substrate reversible Michelis-Menten with Haldane substitution is used as a rate law. <ref name="Palm_thesis_2013> Palm, D.C. (2013). ''The regulatory design of glycogen metabolism in mammalian skeletal muscle'' (Ph.D.). University of Stellenbosch</ref> | Mono substrate reversible Michelis-Menten with Haldane substitution is used as a rate law. <ref name="Palm_thesis_2013> Palm, D.C. (2013). ''The regulatory design of glycogen metabolism in mammalian skeletal muscle'' (Ph.D.). University of Stellenbosch</ref> | ||
| − | <center><math> \frac{\frac{V_{max}}{ | + | <center><math> \frac{\frac{V_{max}}{Km_{Glc6P}}\left( [Glc6P] - \frac{[Glc1P]}{K_{eq}} \right) }{1 + \frac{[Glc6P]}{Km_{Glc6P}} + \frac{[Glc1P]}{Km_{Glc1P}} } </math></center> |
==Parameter values== | ==Parameter values== | ||
| Line 24: | Line 24: | ||
| | | | ||
|- | |- | ||
| − | |<math> | + | |<math>Km_{Glc6P}</math> |
|<math>5.7 \times 10^{-2} </math> <ref name="gao_2004">Gao H & Leary JA (2004). ''Kinetic measurements of phosphoglucomutase by direct analysis of glucose-1-phosphate and glucose-6-phosphate using ion/molecule reactions and Fourier transform ion cyclotron resonance mass spectrometry.'' Anal Biochem 329, 269–275.</ref> | |<math>5.7 \times 10^{-2} </math> <ref name="gao_2004">Gao H & Leary JA (2004). ''Kinetic measurements of phosphoglucomutase by direct analysis of glucose-1-phosphate and glucose-6-phosphate using ion/molecule reactions and Fourier transform ion cyclotron resonance mass spectrometry.'' Anal Biochem 329, 269–275.</ref> | ||
|mM | |mM | ||
| Line 31: | Line 31: | ||
|- | |- | ||
|<math>Km_{Glc1P}</math> | |<math>Km_{Glc1P}</math> | ||
| − | |<math>1.05 \ | + | |<math>0.01054 </math> <ref name="gao_2004">Gao H & Leary JA (2004). ''Kinetic measurements of phosphoglucomutase by direct analysis of glucose-1-phosphate and glucose-6-phosphate using ion/molecule reactions and Fourier transform ion cyclotron resonance mass spectrometry.'' Anal Biochem 329, 269–275.</ref> |
| + | |mM | ||
| + | |Rabbit muscle | ||
| + | | | ||
| + | |} | ||
| + | |||
| + | ==Parameters with uncertainty== | ||
| + | * The value of <math>Km_{Glc6P}</math> using traditional meethods vary in between 0.03 - 0.05 mM. Using these two values as the maximum and the minimum we calculate the mean and standard deviation as <math>0.04 \pm 0.005</math> | ||
| + | |||
| + | |||
| + | {|class="wikitable" | ||
| + | ! Parameter | ||
| + | ! Value | ||
| + | ! Units | ||
| + | ! Organism | ||
| + | ! Remarks | ||
| + | |- | ||
| + | |<math>V_{max}</math> | ||
| + | |<math>2078</math> <ref name = "albe_1990">Albe KR, Butler MH & Wright BE (1990). ''Cellular concentrations of enzymes and their substrates''. J Theor Biol 143, 163–195. </ref> | ||
| + | |<math>\text{mM min}^{-1}</math> | ||
| + | |Rabbit muscle | ||
| + | | | ||
| + | |- | ||
| + | |<math>Km_{Glc6P}</math> | ||
| + | |<math>0.04 \pm 0.005</math> | ||
| + | |mM | ||
| + | |Rabbit muscle | ||
| + | | | ||
| + | |- | ||
| + | |<math>Km_{Glc1P}</math> | ||
| + | |<math>0.01054 \pm 0.0009 </math> <ref name="gao_2004">Gao H & Leary JA (2004). ''Kinetic measurements of phosphoglucomutase by direct analysis of glucose-1-phosphate and glucose-6-phosphate using ion/molecule reactions and Fourier transform ion cyclotron resonance mass spectrometry.'' Anal Biochem 329, 269–275.</ref> | ||
|mM | |mM | ||
|Rabbit muscle | |Rabbit muscle | ||
Revision as of 10:47, 8 May 2014
Phosphoglucomutase (PGLM) facilitates the interconversion of glucose 1-phosphate and glucose 6-phosphate by transfering a phosphate group on an α-D-glucose monomer from the 1' to the 6' position in the forward direction or the 6' to the 1' position in the reverse direction.
Contents
Chemical reaction
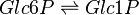
Rate equation
Mono substrate reversible Michelis-Menten with Haldane substitution is used as a rate law. [1]
![\frac{\frac{V_{max}}{Km_{Glc6P}}\left( [Glc6P] - \frac{[Glc1P]}{K_{eq}} \right) }{1 + \frac{[Glc6P]}{Km_{Glc6P}} + \frac{[Glc1P]}{Km_{Glc1P}} }](/wiki/images/math/5/d/c/5dc0ddf39eda6893e8c6161414e55555.png)
Parameter values
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|
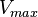
|
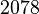 [2] [2]
|

|
Rabbit muscle | |
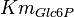
|
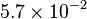 [3] [3]
|
mM | Rabbit muscle | |
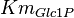
|
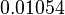 [3] [3]
|
mM | Rabbit muscle |
Parameters with uncertainty
- The value of
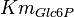 using traditional meethods vary in between 0.03 - 0.05 mM. Using these two values as the maximum and the minimum we calculate the mean and standard deviation as
using traditional meethods vary in between 0.03 - 0.05 mM. Using these two values as the maximum and the minimum we calculate the mean and standard deviation as 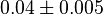
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|
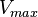
|
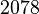 [2] [2]
|

|
Rabbit muscle | |
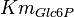
|
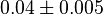
|
mM | Rabbit muscle | |
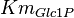
|
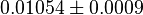 [3] [3]
|
mM | Rabbit muscle |
References
- ↑ Palm, D.C. (2013). The regulatory design of glycogen metabolism in mammalian skeletal muscle (Ph.D.). University of Stellenbosch
- ↑ 2.0 2.1 Albe KR, Butler MH & Wright BE (1990). Cellular concentrations of enzymes and their substrates. J Theor Biol 143, 163–195.
- ↑ 3.0 3.1 3.2 Gao H & Leary JA (2004). Kinetic measurements of phosphoglucomutase by direct analysis of glucose-1-phosphate and glucose-6-phosphate using ion/molecule reactions and Fourier transform ion cyclotron resonance mass spectrometry. Anal Biochem 329, 269–275.