Difference between revisions of "3-phosphoglycerate mutase"
(→Parameters with uncertainty) |
|||
| Line 38: | Line 38: | ||
*Three reported values of <math>Km_{3PG}</math> are considered. 0.19 <ref name="Hernandez2011"></ref>, 0.5<ref name ="Hernandez_2009">Marin-Hernandez, A., Gallardo-Perez, J. C., Ralph, S. J., Rodriguez-Enriquez, S. & Moreno-Sanchez, R. (2009), ''HIF-1α modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms.'' Mini-Rev. Med. Chem. 9, 1084–1101</ref> and 0.4<ref name="Atauri_2005">de Atauri, P.; Repiso, A.; Oliva, B.; Vives-Corrons, J.L.; Climent, F.; Carreras, J. (2005), ''Characterization of the first described mutation of human red blood cell phosphoglycerate mutase'', Biochim. Biophys. Acta 1740, 403-410</ref>. | *Three reported values of <math>Km_{3PG}</math> are considered. 0.19 <ref name="Hernandez2011"></ref>, 0.5<ref name ="Hernandez_2009">Marin-Hernandez, A., Gallardo-Perez, J. C., Ralph, S. J., Rodriguez-Enriquez, S. & Moreno-Sanchez, R. (2009), ''HIF-1α modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms.'' Mini-Rev. Med. Chem. 9, 1084–1101</ref> and 0.4<ref name="Atauri_2005">de Atauri, P.; Repiso, A.; Oliva, B.; Vives-Corrons, J.L.; Climent, F.; Carreras, J. (2005), ''Characterization of the first described mutation of human red blood cell phosphoglycerate mutase'', Biochim. Biophys. Acta 1740, 403-410</ref>. | ||
| − | * Two values of <math>Km_{3PG}</math> are found for Human cells. They are reported as 0.28<ref name ="Hernandez_2009"></ref> and 0.12 | + | * Two values of <math>Km_{3PG}</math> are found for Human cells. They are reported as 0.28<ref name ="Hernandez_2009"></ref> and 0.12<ref name="Hernandez2011"></ref>. Averaging them gives mean value of 0.2 and 0.08 |
{|class="wikitable" | {|class="wikitable" | ||
| Line 62: | Line 62: | ||
|- | |- | ||
|<math>Km_{2PG}</math> | |<math>Km_{2PG}</math> | ||
| − | |0. | + | |<math>0.2 \pm 0.08</math> |
|mM | |mM | ||
|} | |} | ||
Revision as of 14:32, 29 April 2014
Phosphoglycerate mutase (PGAM) is an enzyme that catalyzes the internal transfer of a phosphate group from C-3 to C-2 which results in the conversion of 3-phosphoglycerate (3PG) to 2-phosphoglycerate (2PG).
Contents
Chemical equation
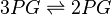
Rate equation
Mono-substrate reversible Michaelis-Menten equation is used. [1]
![\frac{V_{mf}\frac{[3PG]}{Km_{3PG}}-V_{mr}\frac{[2PG]}{Km_{2PG}}}{1 + \frac{[3PG]}{Km_{3PG}} + \frac{[2PG]}{Km_{2PG}}}](/wiki/images/math/6/f/e/6fed036845e1a93d67d5db4525e750af.png)
Parameter values
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
0.94 [2] | 
|
HeLa cell line | |

|
0.36 [2] | 
| ||
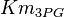
|
0.19[1] | mM | ||
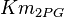
|
0.12[1] | mM |
Parameters with uncertainty
- Two values of
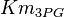 are found for Human cells. They are reported as 0.28[3] and 0.12[1]. Averaging them gives mean value of 0.2 and 0.08
are found for Human cells. They are reported as 0.28[3] and 0.12[1]. Averaging them gives mean value of 0.2 and 0.08
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
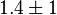 [2] [2]
|

|
||

|
0.36 [2] | 
| ||
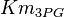
|
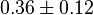
|
mM | ||
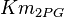
|
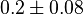
|
mM |
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S et al (2011) Modeling cancer glycolysis. Biochim Biophys Acta 1807:755–767 (doi)
- ↑ 2.0 2.1 2.2 2.3 Marín-Hernández A , Rodríguez-Enríquez S, Vital-González P A, et al. (2006). Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J., 273 , pp. 1975–1988(doi)
- ↑ 3.0 3.1 Marin-Hernandez, A., Gallardo-Perez, J. C., Ralph, S. J., Rodriguez-Enriquez, S. & Moreno-Sanchez, R. (2009), HIF-1α modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini-Rev. Med. Chem. 9, 1084–1101
- ↑ de Atauri, P.; Repiso, A.; Oliva, B.; Vives-Corrons, J.L.; Climent, F.; Carreras, J. (2005), Characterization of the first described mutation of human red blood cell phosphoglycerate mutase, Biochim. Biophys. Acta 1740, 403-410