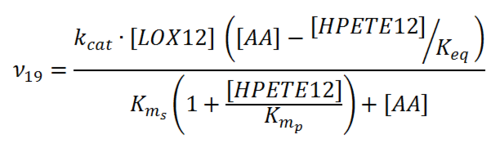Difference between revisions of "Transformation of AA to 12-HPETE"
(→Enzyme Parameters) |
|||
| Line 24: | Line 24: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 33: | Line 34: | ||
pH: 7.4 | pH: 7.4 | ||
Temperature: 37°C. | Temperature: 37°C. | ||
| + | |2048 | ||
|<ref name="Lagarde1984"> [http://www.ncbi.nlm.nih.gov/pubmed/6433902 Lagarde M. "Subcellular localization and some properties of lipoxygenase activity in human blood platelets.'' Biochem J. 1984 Sep 1;222(2):495-500.]</ref> | |<ref name="Lagarde1984"> [http://www.ncbi.nlm.nih.gov/pubmed/6433902 Lagarde M. "Subcellular localization and some properties of lipoxygenase activity in human blood platelets.'' Biochem J. 1984 Sep 1;222(2):495-500.]</ref> | ||
|- | |- | ||
| Line 42: | Line 44: | ||
pH: 7 | pH: 7 | ||
Temperature: 24 | Temperature: 24 | ||
| + | |512 | ||
|<ref name="Hada1991"> [http://www.ncbi.nlm.nih.gov/pubmed/1851637 Hada T. "Catalytic properties of human platelet 12-lipoxygenase as compared with the enzymes of other origins.'' Biochim Biophys Acta. 1991 Apr 24;1083(1):89-93.]</ref> | |<ref name="Hada1991"> [http://www.ncbi.nlm.nih.gov/pubmed/1851637 Hada T. "Catalytic properties of human platelet 12-lipoxygenase as compared with the enzymes of other origins.'' Biochim Biophys Acta. 1991 Apr 24;1083(1):89-93.]</ref> | ||
|- | |- | ||
| Line 51: | Line 54: | ||
pH: 8 | pH: 8 | ||
Temperature: 37 | Temperature: 37 | ||
| + | |256 | ||
|<ref name="Chen1993"> [http://www.ncbi.nlm.nih.gov/pubmed/8319693 Chen X. S. "Purification and characterization of recombinant histidine-tagged human platelet 12-lipoxygenase expressed in a baculovirus/insect cell system.'' Eur J Biochem. 1993 Jun 15;214(3):845-52.]</ref> | |<ref name="Chen1993"> [http://www.ncbi.nlm.nih.gov/pubmed/8319693 Chen X. S. "Purification and characterization of recombinant histidine-tagged human platelet 12-lipoxygenase expressed in a baculovirus/insect cell system.'' Eur J Biochem. 1993 Jun 15;214(3):845-52.]</ref> | ||
|- | |- | ||
| Line 60: | Line 64: | ||
pH: 7.4 | pH: 7.4 | ||
Temperature: 37 | Temperature: 37 | ||
| + | |2048 | ||
|<ref name="Romano1993"> [http://www.ncbi.nlm.nih.gov/pubmed/8250832 Romano M. "Lipoxin synthase activity of human platelet 12-lipoxygenase.'' Biochem J. 1993 Nov 15;296 ( Pt 1):127-33.]</ref> | |<ref name="Romano1993"> [http://www.ncbi.nlm.nih.gov/pubmed/8250832 Romano M. "Lipoxin synthase activity of human platelet 12-lipoxygenase.'' Biochem J. 1993 Nov 15;296 ( Pt 1):127-33.]</ref> | ||
|- | |- | ||
| Line 92: | Line 97: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 101: | Line 107: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 25 | Temperature: 25 | ||
| + | |256 | ||
|<ref name="Wecksler2009"> [www.ncbi.nlm.nih.gov/pubmed/19469483 Wecksler A. "Mechanistic Investigations of Human Reticulocyte 15- and Platelet 12-Lipoxygenases with Arachidonic Acid'' Biochemistry, 2009, 48 (26), pp 6259–6267]</ref> | |<ref name="Wecksler2009"> [www.ncbi.nlm.nih.gov/pubmed/19469483 Wecksler A. "Mechanistic Investigations of Human Reticulocyte 15- and Platelet 12-Lipoxygenases with Arachidonic Acid'' Biochemistry, 2009, 48 (26), pp 6259–6267]</ref> | ||
|- | |- | ||
| Line 110: | Line 117: | ||
pH: 7.4 | pH: 7.4 | ||
Temperature: 37 | Temperature: 37 | ||
| + | |1024 | ||
|<ref name="Richards1997"> [http://pubs.acs.org/doi/abs/10.1021/bi963051a Richards K. "Leukocyte 12-Lipoxygenase: Expression, Purification, and Investigation of the Role of Methionine Residues in Turnover-Dependent Inactivation and 5,8,11,14-Eicosatetraynoic Acid Inhibition'' Biochemistry, 1997, 36 (22), pp 6692–6699]</ref> | |<ref name="Richards1997"> [http://pubs.acs.org/doi/abs/10.1021/bi963051a Richards K. "Leukocyte 12-Lipoxygenase: Expression, Purification, and Investigation of the Role of Methionine Residues in Turnover-Dependent Inactivation and 5,8,11,14-Eicosatetraynoic Acid Inhibition'' Biochemistry, 1997, 36 (22), pp 6692–6699]</ref> | ||
|- | |- | ||
| Line 131: | Line 139: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 140: | Line 149: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | |<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | ||
|- | |- | ||
| Line 149: | Line 159: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
|- | |- | ||
| Line 158: | Line 169: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
|- | |- | ||
| Line 167: | Line 179: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
|- | |- | ||
| Line 188: | Line 201: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 199: | Line 213: | ||
pH: 7.3 | pH: 7.3 | ||
ionic strength: 0.25 | ionic strength: 0.25 | ||
| + | |64 | ||
|<ref name="MetaCyc”>[http://metacyc.org/META/NEW-IMAGE?type=REACTION&object=ARACHIDONATE-12-LIPOXYGENASE-RXN Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471]</ref> | |<ref name="MetaCyc”>[http://metacyc.org/META/NEW-IMAGE?type=REACTION&object=ARACHIDONATE-12-LIPOXYGENASE-RXN Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471]</ref> | ||
|} | |} | ||
Revision as of 10:56, 22 May 2019
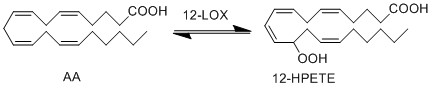
LOX enzymes oxidise AA to generate hydroxy fatty acids. In the skin, the 12-LOX isoform of LOX is more active than others. This isoform is highly expressed in the resident cells of both compartments, epidermal keratinocytes and dermal fibroblasts (Dowd, Kobza Black et al. 1985). The 12- LOX enzyme catalyses the addition of O2 at the C-12 position of AA, producing 12-HPETE.
Contents
Reaction
Chemical equation
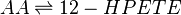
Rate equation
Enzyme Parameters
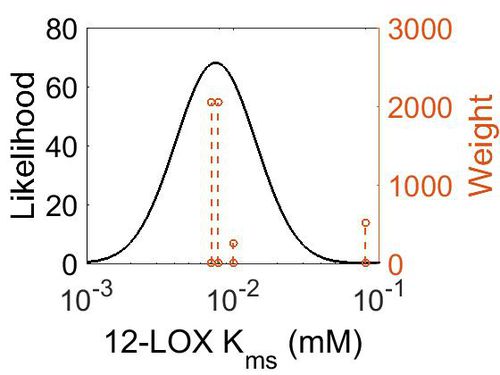
Kms
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 7.20E-03 | 
|
Human | Expression Vector: Platelet
Enzyme: 12-Lipoxygenase pH: 7.4 Temperature: 37°C. |
2048 | [1] |
| 8.00E-02 | 
|
Human | Expression Vector: Platelet
Enzyme: 12-Lipoxygenase pH: 7 Temperature: 24 |
512 | [2] |
| 1.00E-02 | 
|
Human Platlet | Expression Vector: Baculovirus
Enzyme: 12-Lipoxygenase pH: 8 Temperature: 37 |
256 | [3] |
| 7.90E-03 ± 8.00E-04 | 
|
Human | Expression Vector: Platelets
Enzyme:12-Lipoxygenase pH: 7.4 Temperature: 37 |
2048 | [4] |
| Mode (mM) | Confidence Interval | Location parameter (σ) | Scale parameter (σ) |
|---|---|---|---|
| 7.60E-03 | 4.34E+00 | -4.48E+00 | 6.30E-01 |
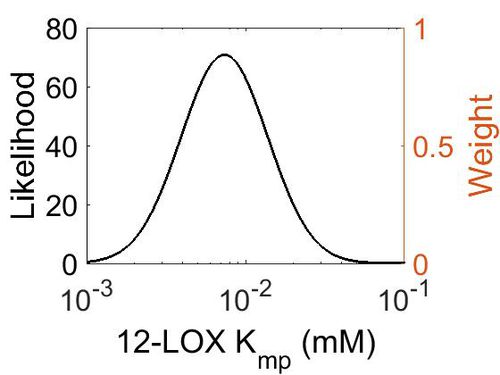
Kmp
This is a “Dependent parameter”, meaning that the log-normal distribution for this parameter was calculated using multivariate distributions (this is discussed in detail here). As a result, no confidence interval factor or literature values were cited for this parameter.
| Mode (mM) | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|
| 7.30E-03 | -4.52E+00 | 6.28E-01 |
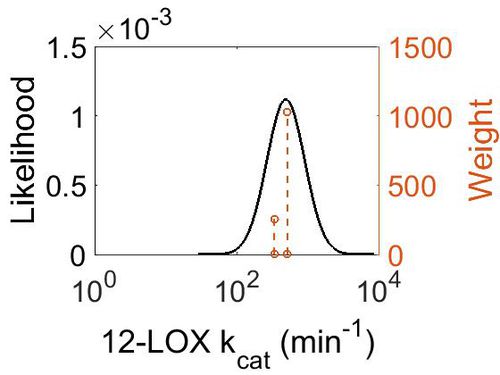
kcat
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 336 ± 12 | per minute | Human | Expression Vector: Human reticulocyte
Enzyme: 15-lipoxygenase-1 pH: 7.5 Temperature: 25 |
256 | [5] |
| 504 | per minute | Wild Boar | Expression Vector: E. coli.
Enzyme: 12-Lipoxygenase pH: 7.4 Temperature: 37 |
1024 | [6] |
| Mode (min-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 4.87E+02 | 1.20E+00 | 6.22E+00 | 1.80E-01 |
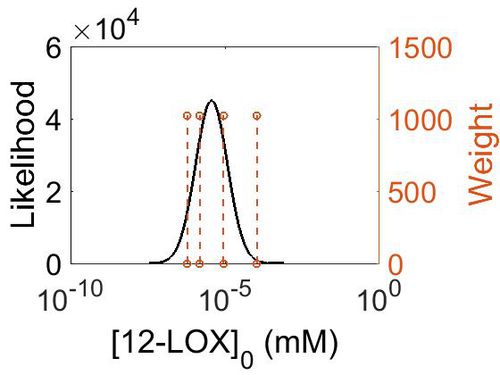
Enzyme concentration
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 19.8 | 
|
Human | Expression Vector: Spleen
Enzyme: 12-LOX pH: 7.5 Temperature: 37 °C |
1024 | [7] |
| 1.60 | 
|
Human | Expression Vector: Liver
Enzyme: 12-LOX pH: 7.5 Temperature: 37 °C |
1024 | [8] |
| 0.28 | 
|
Human | Expression Vector: Gut
Enzyme: 12-LOX pH: 7.5 Temperature: 37 °C |
1024 | [8] |
| 0.11 | 
|
Human | Expression Vector: Pancreas
Enzyme: 12-LOX pH: 7.5 Temperature: 37 °C |
1024 | [8] |
| Mode (mM) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 4.98E-01 | 7.23E+00 | 7.12E-01 | 1.19E+00 |
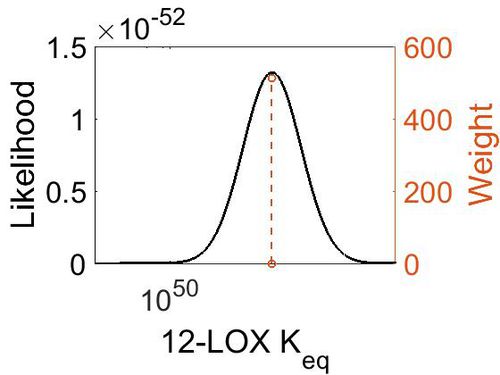
Keq
| Gibbs Free Energy Change | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| (-69.979996) | kcal/mol | Not stated | Estimated
Enzyme: 12-LOX Substrate: Arachidonate Product: 12-HPETE pH: 7.3 ionic strength: 0.25 |
64 | [9] |
| Mode | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 2.27E+51 | 1.00E+01 | 1.19E+02 | 8.90E-01 |
References
- ↑ Lagarde M. "Subcellular localization and some properties of lipoxygenase activity in human blood platelets. Biochem J. 1984 Sep 1;222(2):495-500.
- ↑ Hada T. "Catalytic properties of human platelet 12-lipoxygenase as compared with the enzymes of other origins. Biochim Biophys Acta. 1991 Apr 24;1083(1):89-93.
- ↑ Chen X. S. "Purification and characterization of recombinant histidine-tagged human platelet 12-lipoxygenase expressed in a baculovirus/insect cell system. Eur J Biochem. 1993 Jun 15;214(3):845-52.
- ↑ Romano M. "Lipoxin synthase activity of human platelet 12-lipoxygenase. Biochem J. 1993 Nov 15;296 ( Pt 1):127-33.
- ↑ [www.ncbi.nlm.nih.gov/pubmed/19469483 Wecksler A. "Mechanistic Investigations of Human Reticulocyte 15- and Platelet 12-Lipoxygenases with Arachidonic Acid Biochemistry, 2009, 48 (26), pp 6259–6267]
- ↑ Richards K. "Leukocyte 12-Lipoxygenase: Expression, Purification, and Investigation of the Role of Methionine Residues in Turnover-Dependent Inactivation and 5,8,11,14-Eicosatetraynoic Acid Inhibition Biochemistry, 1997, 36 (22), pp 6692–6699
- ↑ M. Wilhelm Mass-spectrometry-based draft of the human proteome Nature, 2014 509, 582–587
- ↑ 8.0 8.1 8.2 M. Kim A draft map of the human proteome Nature, 2014 509, 575–581
- ↑ Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471