Difference between revisions of "Pyruvate kinase"
| Line 5: | Line 5: | ||
==Rate equation== | ==Rate equation== | ||
| − | The rate equation is represented by the allosteric regualation model of [http://en.wikipedia.org/wiki/MWC_model Monod, Wyman and Changeux] (MWS). [http://en.wikipedia.org/wiki/Fructose_1,6-bisphosphatase Fru1,6BP] and [http://en.wikipedia.org/wiki/Serine Serine] are activators and [http://en.wikipedia.org/wiki/Adenosine_triphosphate ATP] is inhibiting. Simple [http://en.wikipedia.org/wiki/Michaelis%E2%80%93Menten_kinetics Micahelis-Menten kinetics] (Briggs Haldane) is used for ADP and reverse reaction | + | The rate equation is represented by the allosteric regualation model of [http://en.wikipedia.org/wiki/MWC_model Monod, Wyman and Changeux] (MWS). [http://en.wikipedia.org/wiki/Fructose_1,6-bisphosphatase Fru1,6BP] and [http://en.wikipedia.org/wiki/Serine Serine] are activators and [http://en.wikipedia.org/wiki/Adenosine_triphosphate ATP] is inhibiting. Simple [http://en.wikipedia.org/wiki/Michaelis%E2%80%93Menten_kinetics Micahelis-Menten kinetics] (Briggs Haldane) is used for ADP and reverse reaction <ref name="MWC_1965"> Monod J, Wyman J, Changeux J-P (1965) On the Nature of Allosteric Transitions: A Plausible Model . Journal of Molecular Biology 12:88–118 ([http://dx.doi.org/10.1016/S0022-2836(65)80285-6 doi]) </ref> |
<center><math>V_m \left( \left(\frac{\frac{[ADP]}{K_{ADP}}}{1+\frac{[ADP]}{K_{ADP}}}\right) \left( \frac{\frac{[PEP]}{K_{PEP}}\left( 1+\frac{[PEP]}{K_{PEP}} \right)^3 }{ \frac{L \left( 1 + \frac{[ATP]}{Ki_{ADP}} \right)^4 }{ \left( 1 + \frac{[SER]}{Ka_{SER}} \right)^4 \left( 1 + \frac{F1,6BP}{Ka_{F1,6BP}} \right)^4 } + \left( 1 + \frac{[PEP]}{K_{PEP}} \right)^4} \right) - \left( \frac{\frac{[ATP][PYR]}{K_{ATP}K_{PYR}K_{eq}}}{\frac{[ATP]}{K_{ATP}} + \frac{[PYR]}{K_{PYR}} + \frac{[ATP][PYR]}{K_{ATP}K_{PYR} } + 1} \right) \right)</math></center> | <center><math>V_m \left( \left(\frac{\frac{[ADP]}{K_{ADP}}}{1+\frac{[ADP]}{K_{ADP}}}\right) \left( \frac{\frac{[PEP]}{K_{PEP}}\left( 1+\frac{[PEP]}{K_{PEP}} \right)^3 }{ \frac{L \left( 1 + \frac{[ATP]}{Ki_{ADP}} \right)^4 }{ \left( 1 + \frac{[SER]}{Ka_{SER}} \right)^4 \left( 1 + \frac{F1,6BP}{Ka_{F1,6BP}} \right)^4 } + \left( 1 + \frac{[PEP]}{K_{PEP}} \right)^4} \right) - \left( \frac{\frac{[ATP][PYR]}{K_{ATP}K_{PYR}K_{eq}}}{\frac{[ATP]}{K_{ATP}} + \frac{[PYR]}{K_{PYR}} + \frac{[ATP][PYR]}{K_{ATP}K_{PYR} } + 1} \right) \right)</math></center> | ||
| + | |||
| + | ==Parameter values== | ||
| + | |||
| + | ==Alternative parameter values== | ||
| + | |||
| + | ==References== | ||
| + | <references/> | ||
Revision as of 18:30, 27 February 2014
Pyruvate kinase is a transferase enzyme that catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to ADP, yielding one molecule of pyruvate and one molecule of ATP.
Contents
Chemical reaction
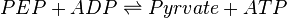
Rate equation
The rate equation is represented by the allosteric regualation model of Monod, Wyman and Changeux (MWS). Fru1,6BP and Serine are activators and ATP is inhibiting. Simple Micahelis-Menten kinetics (Briggs Haldane) is used for ADP and reverse reaction [1]
![V_m \left( \left(\frac{\frac{[ADP]}{K_{ADP}}}{1+\frac{[ADP]}{K_{ADP}}}\right) \left( \frac{\frac{[PEP]}{K_{PEP}}\left( 1+\frac{[PEP]}{K_{PEP}} \right)^3 }{ \frac{L \left( 1 + \frac{[ATP]}{Ki_{ADP}} \right)^4 }{ \left( 1 + \frac{[SER]}{Ka_{SER}} \right)^4 \left( 1 + \frac{F1,6BP}{Ka_{F1,6BP}} \right)^4 } + \left( 1 + \frac{[PEP]}{K_{PEP}} \right)^4} \right) - \left( \frac{\frac{[ATP][PYR]}{K_{ATP}K_{PYR}K_{eq}}}{\frac{[ATP]}{K_{ATP}} + \frac{[PYR]}{K_{PYR}} + \frac{[ATP][PYR]}{K_{ATP}K_{PYR} } + 1} \right) \right)](/wiki/images/math/8/f/8/8f80463ba7879b64d7b1bf290f1e5bc8.png)