Difference between revisions of "UDPG-pyrophosphorylase"
(→Parameter values) |
(→Parameter values) |
||
| Line 19: | Line 19: | ||
|200 <ref name = "villar_1960"> Villar-Palasi C & Larner J (1960). ''Levels of activity of the enzymes of the glycogen cycle in rat tissues''. Arch Biochem Biophys 86, 270–273.</ref> | |200 <ref name = "villar_1960"> Villar-Palasi C & Larner J (1960). ''Levels of activity of the enzymes of the glycogen cycle in rat tissues''. Arch Biochem Biophys 86, 270–273.</ref> | ||
|<math>min^{-1}</math> | |<math>min^{-1}</math> | ||
| − | |Recombinant, human muscle | + | |rowspan="6"|Recombinant, human muscle |
| | | | ||
|- | |- | ||
| Line 25: | Line 25: | ||
|0.4 <ref name="duggleby_1996"> Duggleby RG, Chao YC, Huang JG, Peng HL & Chang HY (1996). ''Sequence differences between human muscle and liver cDNAs for UDPglucose pyrophosphorylase and kinetic properties of the recombinant enzymes expressed in Escherichia coli''. Eur J Biochem 235, 173–179. </ref> | |0.4 <ref name="duggleby_1996"> Duggleby RG, Chao YC, Huang JG, Peng HL & Chang HY (1996). ''Sequence differences between human muscle and liver cDNAs for UDPglucose pyrophosphorylase and kinetic properties of the recombinant enzymes expressed in Escherichia coli''. Eur J Biochem 235, 173–179. </ref> | ||
|mM | |mM | ||
| − | |||
| | | | ||
|- | |- | ||
| Line 31: | Line 30: | ||
|0.92 <ref name="duggleby_1996"></ref> | |0.92 <ref name="duggleby_1996"></ref> | ||
|mM | |mM | ||
| − | |||
| | | | ||
|- | |- | ||
| Line 37: | Line 35: | ||
|<math> 6.3 \times 10^{-2} </math> <ref name="duggleby_1996"></ref> | |<math> 6.3 \times 10^{-2} </math> <ref name="duggleby_1996"></ref> | ||
|mM | |mM | ||
| − | |||
| | | | ||
|- | |- | ||
| Line 43: | Line 40: | ||
|<math>0.38</math><ref name="duggleby_1996"></ref> | |<math>0.38</math><ref name="duggleby_1996"></ref> | ||
|mM | |mM | ||
| − | |||
| | | | ||
|- | |- | ||
| Line 49: | Line 45: | ||
|<math>0.24</math><ref name="duggleby_1996"></ref> | |<math>0.24</math><ref name="duggleby_1996"></ref> | ||
|Dimensionless | |Dimensionless | ||
| − | |||
| | | | ||
|} | |} | ||
Revision as of 10:50, 27 March 2014
This enzyme converts UTP and G1P to UDP-glucose (UDPG) and pyrophosphate (PPi)
Chemical equation
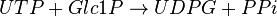
Rate equation
Reversible Bi substrate Michaelis-Menten equation with random binding order is used [1]
![\frac{ \frac{V_{max}}{K_{UTP}K_{Glc1P}} \left( [UTP][Glc1P] - \frac{[UDPG][PPi]}{K_{eq}} \right) }{ \left( 1 + \frac{[UTP]}{K_{UTP}} + \frac{[PPi]}{K_{PPi}} \right) \left( 1 + \frac{[UDPG]}{K_{UDPG}} + \frac{[Glc1P]}{K_{Glc1P}} \right) }](/wiki/images/math/5/c/b/5cb04d31df5c22ab51ad23a54e27d997.png)
Parameter values
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|
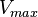
|
200 [2] | 
|
Recombinant, human muscle | |
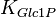
|
0.4 [3] | mM | ||
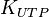
|
0.92 [3] | mM | ||
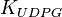
|
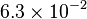 [3] [3]
|
mM | ||
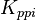
|
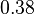 [3] [3]
|
mM | ||

|
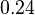 [3] [3]
|
Dimensionless |
References
- ↑ Palm, D.C. (2013). The regulatory design of glycogen metabolism in mammalian skeletal muscle (Ph.D.). University of Stellenbosch
- ↑ Villar-Palasi C & Larner J (1960). Levels of activity of the enzymes of the glycogen cycle in rat tissues. Arch Biochem Biophys 86, 270–273.
- ↑ 3.0 3.1 3.2 3.3 3.4 Duggleby RG, Chao YC, Huang JG, Peng HL & Chang HY (1996). Sequence differences between human muscle and liver cDNAs for UDPglucose pyrophosphorylase and kinetic properties of the recombinant enzymes expressed in Escherichia coli. Eur J Biochem 235, 173–179.