Background Information on GBL system
Contents
Bacterial communication & "Quorum sensing"
Many microorganisms produce antibiotics in order to gain a competitive advantage over other organisms for their survival. However, coordination between members of the population is critical as the end products can be fatal for the colony if produced in an unregulated manner. One prominent mechanism employed is a mechanism of "voting" by members of the colony, by sensing and producing signalling molecules. If a large number of surrounding neigbours are producing the signalling molecules in response to an environmental cue, this is likely to indicate the right moment to produce antibiotics. [1]
These mechanisms have been targeted by synthetic biology whose core aim is the design and engineering of complex biological systems with functionalities that do not exist in nature. In order to accomplish this, novel regulatory circuits need to be developed, which will enable the precise control of gene expression over a wide range of conditions. [2] The bacterium Vibrio fischeri has been in the spotlight in recent years, due to its communication mechanism known as "quorum sensing" (QS). [3] This organism produces acyl homoserine lactone (AHL) as a signal molecule and exports it to the environment. As the colony grows and the cell density increases, the concentration of AHL in the environment also rises, until it reaches a threshold which activates the expression of specific genes responsible for the emission of light.
The system responsible for the production of AHL is composed of two genes and their encoding regulatory proteins LuxI and LuxR. LuxI is the actual synthase of the autoinducer and LuxR, is a repressor belonging to the TetR family. [4] AHL forms a complex with the LuxR protein and together they bind to a short sequence called lux box which both enhances the transcription of LuxI (thus leading to even further accumulation of AHL) and activates the bioluminescence gene cluster. This positive feedback loop leads to a behaviour similar to a bistable switch, with the system being either in the "on" or in the "off" state, and with a zone of unstable intermediate states between them.
Regulation in Streptomyces coelicolor
Although the quorum sensing circuit has been widely employed in synthetic biology with numerous successful applications, [5] [6] [7] it has some important limitations, such as potential crosstalk between different systems due to the promiscuity of the signalling molecules or the promoters, [8] and the problematic implementation in eukaryotic organisms. [9]
A good candidate for this purpose could be the γ-butyrolactone (GBL) signalling circuits of Streptomyces coelicolor. These are Gram-positive, filamentous, soil-dwelling bacteria, which are known as prolific source of secondary metabolites, such as antibiotics (actinorhodin (Act), undecylprodigiosin (Red), methylenomycin (Mmy), and calcium-dependent antibiotic (CDA)). [10] [11] As the end-products can be toxic even to the producing organisms, [12] antibiotic production is carefully coordinated in the bacterial population. One way Streptomyces can regulate secondary metabolite production is through the use of small diffusible molecules, known as γ-butyrolactones, in a manner analogous to acyl homoserine lactone (AHL)-based quorum sensing (QS). [13] At this point, more than fifteen different GBLs have been identified in various Streptomyces species. [12] [14]
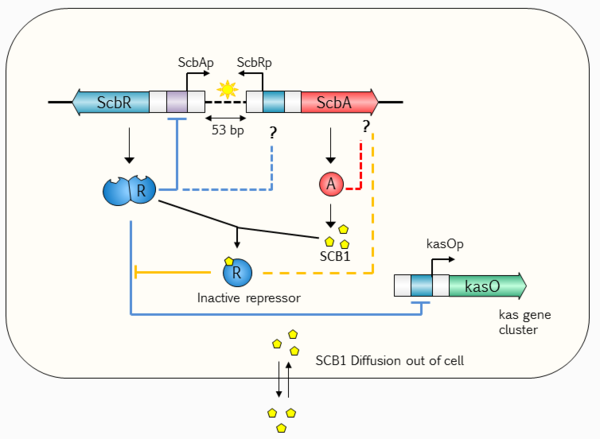
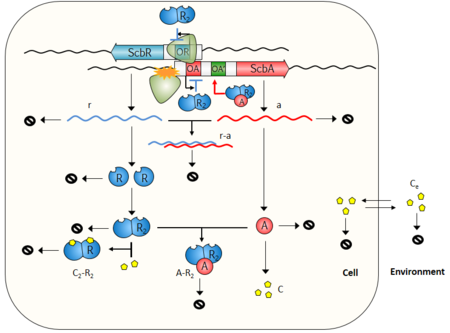
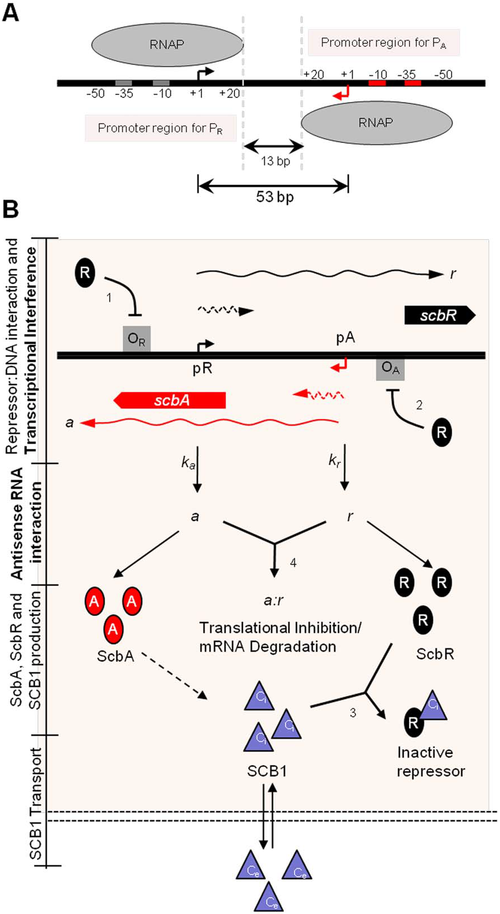
Recent studies and reviews [15] [16] point to the hypothesis that the GBL regulatory system involves a small, yet complex two gene network composed by a synthase (scbA (SCO6266)) and a butyrolactone receptor (scbR (SCO6265)), which governs a potentially bistable switch between the "on" and "off" states of antibiotic production. The scbA gene product is presumed to catalyse the condensation of dihydroxyacetone phosphate with a beta-ketoacid to produce three different butyrolactones (SCB1, SCB2 and SCB3). [12] [17] On the other hand, scbR is a TetR-like [4] DNA-binding protein known to regulate its own transcription and that of scbA. Additionally, ScbR protein has a γ-butyrolactone binding domain at its C-terminal and a DNA binding domain at its N-terminal [18]. This structure allows it to directly regulate the production of a cryptic metabolite and indirectly the production of blue pigmented actinorhodin (Act) and prodigiosins (e.g. undecylprodigiosin, Red). These genes are divergently encoded and their promoter regions overlap 53bp, as has been shown by gel retardation assays and DNase I footprinting studies. [19] Complementary to these findings, the discovery of cis asRNA has also been reported in different studies.[20] [21] [22] The regulatory role of the system's overlapping topology and of the antisense RNA in biological decision making has been demonstrated in number of studies concerning different bacteria, such as E. faecalis [23] and S. enterica. [24] It is therefore possible that the formation of an antisense RNA between the scbA and scbR transcripts could play an important role in the regulation of the GBL system.
Transcription analyses have shown that both genes are mainly active during transition from logarithmic growth to stationary phase. It is presumed that SCBs slowly accumulate into the media and upon reaching a concentration threshold promote a coordinated switch-like transition to antibiotic production by binding to ScbR. However, the mechanism of this network is not fully defined, although several alternative scenarios have been proposed. [12] [1] [25] The complete elucidation of this system could potentially lead to the design of robust and sensitive systems with significant applications as orthologous regulatory circuits in synthetic biology and biotechnology. [2]
Effects of overlapping promoters and Cis-antisense RNA
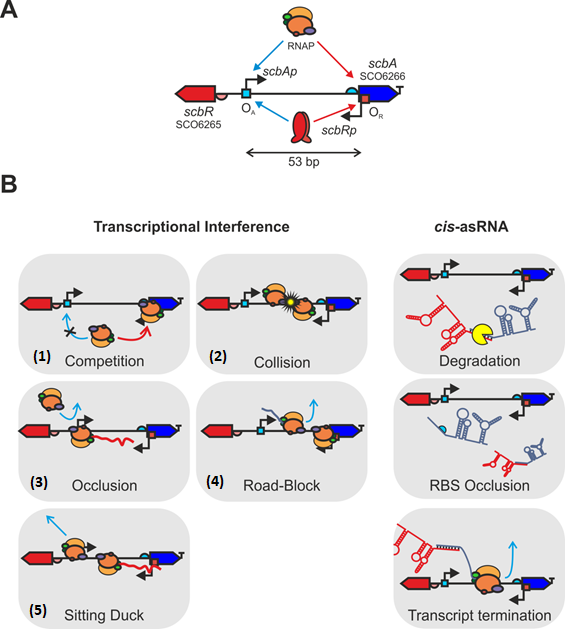
Cis-asRNA transcripts are generally due to the presence of promoters in opposing DNA strands. [26] Base pairing between these RNA molecules can result in either transcript degradation,[27] ribosome binding site (RBS) occlusion or premature transcription termination by destabilisation of the RNA polymerase (RNAP)•RNA complex. [28] [29]
On the other hand, transcriptional interference arises from the suppression of the transcription of one promoter (sensitive promoter, Psens) by the transcriptional machinery of an adjacent promoter (aggressive promoter, Paggr), within this convergent-overlapping architecture.
This interference can occur via five possible scenarios or a combination of them: (1) via promoter competition, where RNAP binding in the aggressive promoter (RNAPaggr) is preferred over binding in the sensitive promoter (RNAPsens); (2) a collision between two opposed elongating RNAPs moving in opposite directions, resulting in premature transcript termination of one or both strands; (3) occlusion of a promoter by the RNAPaggr, which prevents binding and/or recognition of the RNAPsens to its cognate promoter; (4) a blockage of the RNAPsens elongation by the RNAPaggr, which causes premature transcript termination; and (5) removal of promoter-bound complexes by the passage of RNAP from the opposing promoter (Sitting Duck interference). [29]
Previous modelling work and hypotheses
In 2008, Mehra et al. [1] proposed a deterministic model involving a putative heterodimer of the two proteins (ScbR-ScbA) which would bind to a different site upstream of scbA and further activate its transcription. More recently, Chatterjee et al. [25] proposed an alternative model of the system where ScbA only catalyses the SCB biosynthesis. In this model the promoter overlap and antisense RNA formation between scbA and scbR are the sole driving forces of the precise switch-like transition. During both previous modelling efforts on this system, the work was mostly focused on exploring the parameters which induced bistability in the system, without so much considering how closely the model's output matches the experimental data. Finally, in 2001, Takano et al. [19] suggested that the ScbR protein might have a potential dual role in the GBL system. It could potentially suppress the transcription of its own gene but activate the transcription of scbA. This hypothesis has not yet been tested (and validated) neither by experiments nor by computational models.
This system therefore provides a good opportunity for the design and analysis of a model explicitly acknowledging uncertainty which will examine all possible scenarios within a range of reasonable parameters. The ultimate aim of this project was that the improved model would successfully describe the mechanism of the GBL regulatory system and allow reliable predictions of its behaviour. The work in this project was performed in close collaboration with experimental microbiologists who provided their expert advice in the biological aspects, as well as experimental data for the model validation.
Assumptions in the improved model
As the regulatory interactions in the GBL system have not yet been fully elucidated, our aim was to explore all the previously proposed mechanisms (Mehra et al. [1], Chatterjee et al. [25]) under the scope of realistic parameter values retrieved from the literature. In order to achieve this, we designed a unified meta-model that includes all the potential mechanisms and enables their individual or combined use by switching certain reactions "on" and "off". Many aspects of the model are adapted from the quorum sensing model by Weber et al.[30]
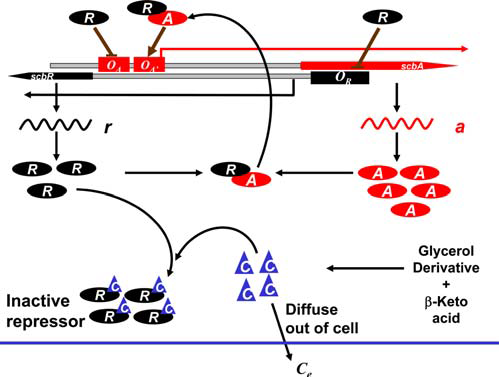
In our model, the ScbR homo-dimer binds to the operators of both scbR and scbA genes and represses their activity. As reported by Bhukya et al.,[31] two ScbR homo-dimers can bind to the operator. When one homo-dimer is bound, the mRNA transcription is already being repressed. As the concentration of ScbR rises, a second homo-dimer may bind to the already suppressed operator and further enhance the suppression of the transcription. ScbA protein (A), through an enzymatic reaction with glycerol derivatives and β-keto acid derivative precursors (S), produces the γ-butyrolactones (C). Our model considers the production of C to be proportional to the concentration of A. γ-butyrolactone (C) then creates a complex with the ScbR protein (C2•R2) and thus effectively deactivates it, enabling further production of ScbA. The signalling molecules (C) diffuse passively between the cells and the environment (Ce) and thus accumulate in the culture medium. The model assumes that internal and external SCBs degrade at the same rate. Additionally, we assume that all molecules are homogeneously distributed both in the cytoplasm and in the medium. DNA duplication, degradation of chemical species and their dilution due to cellular growth are also considered.
State of promoters and transcription
In order to describe the overlapping promoter effects, a mathematical model for overlapping promoters proposed by Bendtsen et al.[32] was employed as described in pages Transcription of r and Transcription of a.
Cell growth and division
As the experimental time simulated is over 60 hours, the effects of cell growth must be taken into consideration in addition to the various regulatory mechanisms. In our model, the number of cells is described by a six-parameter Baranyi–Roberts model,[33] [34] which takes into account the lag phase by using an adjustment function. Detailed information can be found in the page Cellular growth.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 S. Mehra, S. Charaniya, E. Takano, and W.-S. Hu. A bistable gene switch for antibiotic biosynthesis: The butyrolactone regulon in streptomyces coelicolor. PLoS ONE, 3(7), 2008.
- ↑ 2.0 2.1 M. Biarnes-Carrera, R. Breitling, E. Takano Butyrolactone signalling circuits for synthetic biology. Current Opinion in Chemical Biology 28: 91-98, 2015.
- ↑ Z. Li and S. K. Nair, Quorum sensing: how bacteria can coordinate activity and synchronize their response to external signals? Protein Science 21(10): 1403-1417, 2012.
- ↑ 4.0 4.1 Ramos JL, Martínez-Bueno M, Molina-Henares AJ, et al. , The TetR Family of Transcriptional Repressors Microbiol Mol Biol Rev., 69(2): 326–356, 2005.
- ↑ You L., Cox RS 3rd, Weiss R., Arnold FH. Programmed population control by cell-cell communication and regulated killing. Nature, 428(6985):868-71, 2004.
- ↑ Danino T., Mondragón-Palomino O., Tsimring L., Hasty J. A synchronized quorum of genetic clocks Nature, 463(7279):326-30, 2010.
- ↑ Sayut, D. J., Niu, Y., & Sun, L. Construction and enhancement of a minimal genetic AND logic gate. Appl. Environ. Microbiol., 75(3), 637-642, 2009.
- ↑ Wu, F., Menn, D. J., & Wang, X. Quorum-sensing crosstalk driven synthetic circuits: from unimodality to trimodality Chemistry & biology, 21(12), 1629-1638, 2014.
- ↑ Hartmann A., Schikora A. Quorum sensing of bacteria and trans-kingdom interactions of N-acyl homoserine lactones with eukaryotes. Journal of chemical ecology, 38(6), 704-713, 2012.
- ↑ Cimermancic, P., Medema, M. H., Claesen, J., Kurita, K., Brown, L. C. W., Mavrommatis, K., ... & Birren, B. W. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell, 158(2), 412-421, 2014.
- ↑ Price, B., Adamidis, T., Kong, R., & Champness, W. A Streptomyces coelicolor antibiotic regulatory gene, absB, encodes an RNase III homolog. Journal of bacteriology, 181(19), 6142-6151, 1999.
- ↑ 12.0 12.1 12.2 12.3 Takano, E., γ-Butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Current opinion in microbiology, 9(3), 287-294, 2006.
- ↑ Ng, W. L., & Bassler, B. L., Bacterial quorum-sensing network architectures. Annual review of genetics, 43, 197-222, 2009.
- ↑ Kitani, S., Miyamoto, K. T., Takamatsu, S., Herawati, E., Iguchi, H., Nishitomi, K., ... & Nihira, T., Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proceedings of the National Academy of Sciences, 108(39), 16410-16415, 2011.
- ↑ van Wezel, G. P., & McDowall, K. J., The regulation of the secondary metabolism of Streptomyces: new links and experimental advances. Natural product reports, 28(7), 1311-1333, 2011.
- ↑ Liu, G., Chater, K. F., Chandra, G., Niu, G., & Tan, H., Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol. Mol. Biol. Rev., 77(1), 112-143, 2013.
- ↑ Kato, J. Y., Funa, N., Watanabe, H., Ohnishi, Y., & Horinouchi, S., Biosynthesis of γ-butyrolactone autoregulators that switch on secondary metabolism and morphological development in Streptomyces. Proceedings of the National Academy of Sciences, 104(7), 2378-2383, 2007.
- ↑ Natsume, R., Ohnishi, Y., Senda, T., & Horinouchi, S. , Crystal structure of a γ-butyrolactone autoregulator receptor protein in Streptomyces coelicolor A3 (2). Journal of molecular biology, 336(2), 409-419, 2004.
- ↑ 19.0 19.1 E. Takano, R. Chakraburtty, T. Nihira, Y. Yamada, & M. J. Bibb. A complex role for the γ‐butyrolactone SCB1 in regulating antibiotic production in Streptomyces coelicolor A3 (2). Molecular microbiology 41(5): 1015-1028,2001.
- ↑ D'Alia, D., Nieselt, K., Steigele, S., Müller, J., Verburg, I., & Takano, E., Noncoding RNA of glutamine synthetase I modulates antibiotic production in Streptomyces coelicolor A3 (2). Journal of Bacteriology, 192(4), 1160-1164, 2010.
- ↑ Pánek, J., Bobek, J., Mikulík, K., Basler, M., & Vohradský, J., Biocomputational prediction of small non-coding RNAs in Streptomyces. Bmc Genomics, 9(1), 217, 2008.
- ↑ Swiercz, J. P., Bobek, J., Haiser, H. J., Di Berardo, C., Tjaden, B., & Elliot, M. A., Small non-coding RNAs in Streptomyces coelicolor. Nucleic acids research, 36(22), 7240-7251, 2008.
- ↑ Chatterjee, A., Johnson, C. M., Shu, C. C., Kaznessis, Y. N., Ramkrishna, D., Dunny, G. M., & Hu, W. S., Convergent transcription confers a bistable switch in Enterococcus faecalis conjugation. Proceedings of the National Academy of Sciences, 108(23), 9721-9726, 2011.
- ↑ Lee, E. J., & Groisman, E. A., An antisense RNA that governs the expression kinetics of a multifunctional virulence gene. Molecular microbiology, 76(4), 1020-1033, 2010.
- ↑ 25.0 25.1 25.2 25.3 A. Chatterjee, L. Drews, S. Mehra, E. Takano, Y.N. Kaznessis, and W.-S. Hu. Convergent transcription in the butyrolactone regulon in streptomyces coelicolor confers a bistable genetic switch for antibiotic biosynthesis. PLoS ONE, 6(7), 2011.
- ↑ Courtney, C. M. & Chatterjee, A. cis-Antisense RNA and Transcriptional Interference : Coupled layers of gene regulation. J. Gene Ther. 2, 9–15, 2014
- ↑ Arraiano, C. M., Andrade, J. M., Domingues, S., Guinote, I. B., Malecki, M., Matos, R. G., ... & Silva, I. J., The critical role of RNA processing and degradation in the control of gene expression. FEMS microbiology reviews, 34(5), 883-923, 2010
- ↑ Brantl, S., Regulatory mechanisms employed by cis-encoded antisense RNAs. Current opinion in microbiology, 10(2), 102-109, 2007
- ↑ 29.0 29.1 29.2 Biarnes-Carrera, M., Evaluation of a convergent promoter overlap as a mechanism to regulate γ-butyrolactone production (Chapter from PhD Thesis), 2018
- ↑ Weber, M., & Buceta, J., Dynamics of the quorum sensing switch: stochastic and non-stationary effects. BMC systems biology, 7(1), 6, 2013.
- ↑ Bhukya, H., Bhujbalrao, R., Bitra, A., & Anand, R., Structural and functional basis of transcriptional regulation by TetR family protein CprB from S. coelicolor A3 (2). Nucleic acids research, 42(15), 10122-10133, 2014.
- ↑ Bendtsen KM, Erdőssy J, Csiszovszki Z, et al. Direct and indirect effects in the regulation of overlapping promoters. Nucleic Acids Research. 2011;39(16):6879-6885. doi:10.1093/nar/gkr390.
- ↑ Baranyi, J. and T. A. Roberts A dynamic approach to predicting bacterial growth in food. International Journal of Food Microbiology 23(3): 277-294, 1994.
- ↑ J. Baranyi, T. A. Roberts, P. McClure A non-autonomous differential equation to model bacterial growth. Food Microbiology 10(1): 43-59, 1993.